Expressed breast milk (maternal and donor)
Objectives
This guideline is intended to ensure that all staff within the Neonatal Managed Clinical Network (West of Scotland) understand their role and responsibilities in supporting parents to feed and care for their baby in ways which support optimum health and well being.
This guidance should be used in conjunction with local feeding policies and guidelines.
Audience
This guideline is applicable to neonatal, paediatric, nursing and midwifery staff working with neonates in the West of Scotland Neonatal Managed Clinical Network.
All staff are expected to comply with this policy.
Definitions:
Fresh breast milk – expressed breast milk which has been expressed and stored in a refrigerator at ≤4°C for no longer than 48 hours (24 h if expressed at home)
Frozen breast milk – expressed breast milk which has been frozen at a temperature of -18°C or lower (NICE 2010)
Donor breast milk – breast milk expressed by a mother that is then processed by a donor milk bank for use by a recipient that is not the mother’s own baby
It is vital for mothers to start to express early and to express frequently and effectively to ensure a good milk supply and establishment of lactation.
- Skin to skin contact or kangaroo mother care (KMC) and massage of the breast, ideally before expressing, will increase hormone levels and milk volumes and should therefore be encouraged regularly. Mothers should be encouraged to be with their babies for as long as, and as often as, they wish.
- Mother’s should initiate breast milk expression shortly after birth. Hand expression should be used to remove colostrum first. Subsequently mothers should express using the “Initiate” setting on the breast pump. Ideally the initiation of expression should start within the first hour of birth unless maternal health issues prevent this happening. If this is unable to occur for practical reasons then expression should commence within a maximum of 6 hours.
- Expression to remove colostrum should occur as frequently as possible allowing no more than a 6 hour gap overnight. Double pumping for 15 minutes 8 times is required for optimal expression.
- Effective expressing is easier when mothers and babies are close to each other. This should be encouraged and supported through practical measures such as comfortable chairs, adequate cot side expressing facilities and equipment and a screen for privacy. Pumping near baby where possible and pumping both breasts simultaneously can also increase milk yield.
- Ensure the pumping funnel is the correct size, has a good seal and fits comfortably and mother is clear about how to use the pump effectively will also increase yield.
- After Lactogenesis II (milk “comes in”) to increase milk yield, use breast compressions and/or hand expression towards the end of the pumping session when milk flow has decreased to drops.
- When production reaches at least 750-900mls per day the milk cells are likely to have been sufficiently primed and lactation will become established. This is usually by day 10 after the birth. The mother can then safely reduce frequency of expressing to 6 – 8 times per day.
- A low milk production requires prompt attention including a review of technique, frequency of expression, equipment, general support and encouragement. Occasionally this will require referral to the Infant Feeding Coordinator.
The aim is not to meet the baby’s needs but to ensure future sufficient lactation. Within a few days many mothers of small babies will be producing much more than the baby initially needs.
Collection of Breast Milk
Premature and ill babies are much more vulnerable to common bacteria and viruses which normally would not cause severe illness. Therefore attention to hygiene is essential:
- Any staff or mothers involved in milk collection or breast pump use need to be very particular about hand washing before handling equipment or milk containers.
- Mothers should have regular showers. The mother may also require to wash more frequently if she is leaking milk or has increased perspiration (common when prolactin levels are high). Bath towels and bra should be changed daily. Fresh breast pads should be used 4 – 6 hourly, or sooner if damp.
- Staff and mothers need to ensure thorough cleaning of work surfaces and breastfeeding equipment.
- All Colostrum/EBM collected should be labelled with date, time expressed and baby’s name, unit/CHI number.
NB- It is important to feed /use the colostrum in the order in which it was expressed. This may be made easier by numbering the syringes as they are filled.
- Do not overfill bottles (50mls max) as this may lead to wastage. Milk expands when frozen.
Hand expressing
- Initially, when hand expressing mothers, should use a single use sterile enteral syringe or galipot to collect colostrum. If colostrum is stored in an enteral syringe it needs to be clearly labelled with baby’s name, unit number/CHI, date and time expressed.
Hand pumps
- Mothers’ own hand pumps should be cleaned and stored in the same way as pump sets and the surface of electric pumps for personal use should also be cleaned and dried in the same way.
- When using a hand pump use sterile bottles provided using a new sterile bottle(s) each time
Electric breast pumps
- Breast pumps for communal use in ward areas should be regularly serviced and checked for faults. A schedule for this should be available for inspection and audited at least annually.
- Communal breast pumps should be checked daily for wear and tear and cleanliness and thoroughly cleaned by a staff member. This should be recorded daily and audited on a regular basis, at least annually.
- Mothers should be instructed to clean the breast pump before and after they have expressed their milk. Suitable cleansing wipes should be available for this.
- When using an electric pump use sterile bottles provided, using a new sterile bottle each time
Following hand or breast pump expression of breast milk the milk collection kits should be thoroughly cleaned by one of the following methods, as per your local guideline:
- For babies with a high susceptibility to infection, who are being cared for in a neonatal unit - Rinse the sets thoroughly ensuring all milk debris is removed. First wash with warm soapy water (approx 30o) and then rinse with clean water. After cleaning, an additional method of decontamination should be used. The preferred method is to sterilise the sets using microwave steam sterilisation as this has better quality assurance than chemical sterilisation solutions. Medela Quick Clean Micro-Steam Sterilisation bags are a suitable product. These sterilisation bags may be used by an individual mother for 20 episodes of sterilisation.
Once sterilisation complete, dry with a paper towel and store in either a zip-lock plastic bag or a plastic storage container until next use. In hospital these storage containers must be labelled with the baby’s name, unit and CHI number, date and time.
- For babies who are on the postnatal wards or who have been discharged home.
Option 1. Clean the sets with hot soapy water ensuring all milk debris is removed. The sets should then be rinsed and thoroughly dried with fresh paper towel and dry stored away from sinks to avoid contamination by splashes. In hospital this must be labelled with the baby’s name, unit and CHI number, date and time.
Option 2. If the mother is expressing at home then the sets may be cleaned in a dishwasher. Sets should be placed in the top shelf of the dishwasher. Ideally, a large plastic container and lid, which is washed and dried each time with the pump set, should be used to store the equipment.
Pump sets do not need to changed if being appropriately cleaned and stored unless damaged.
Refrigerator
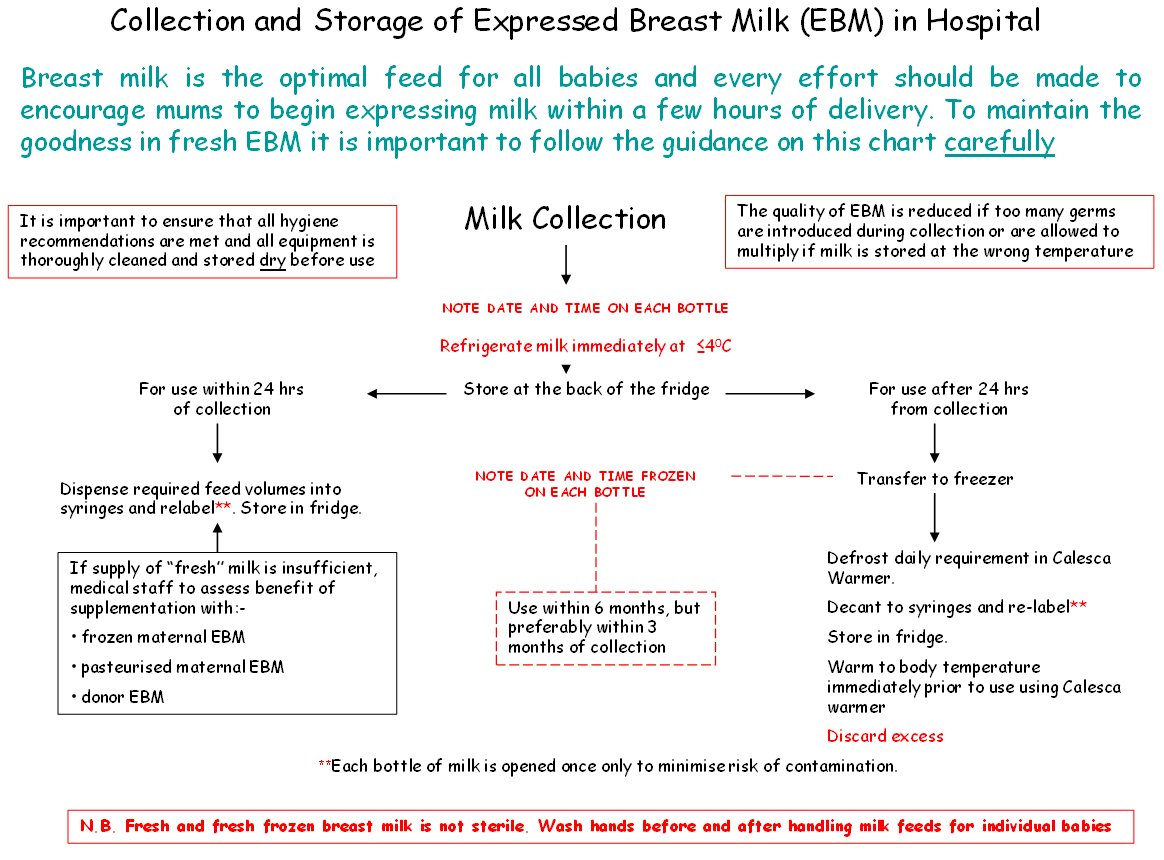
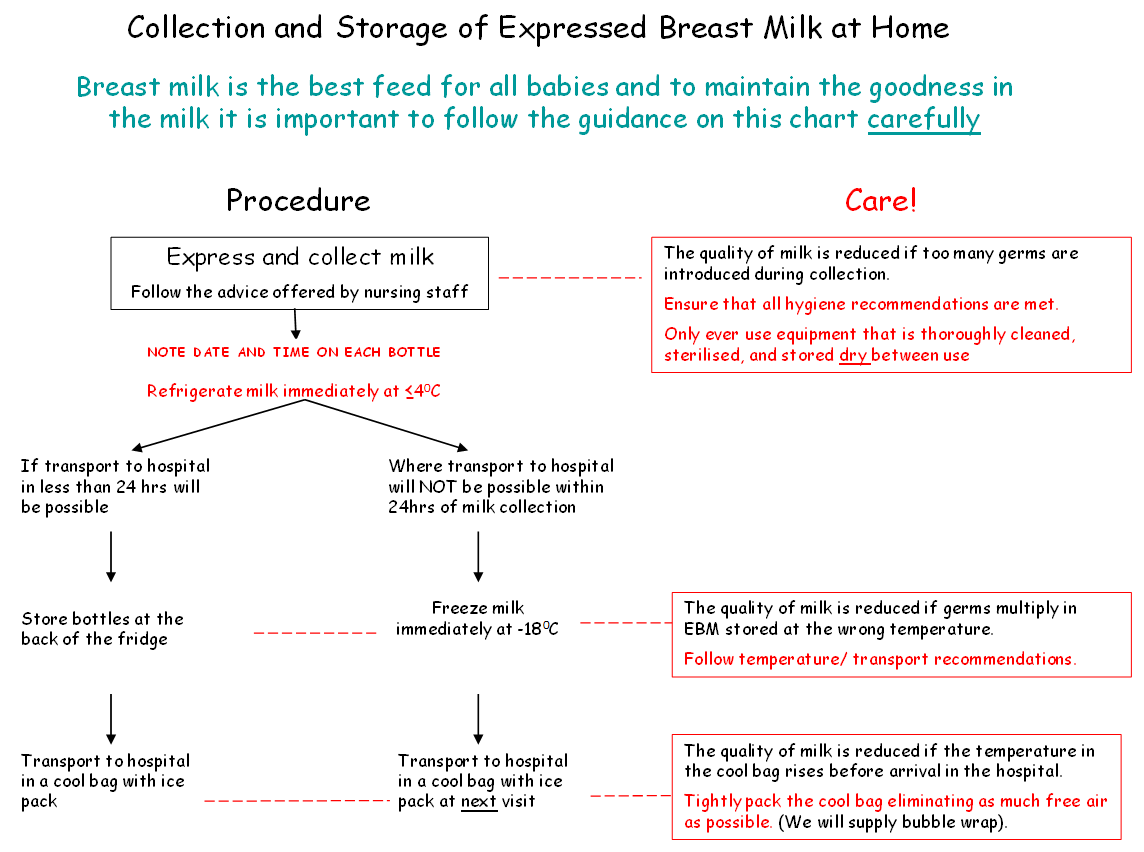
- Expressed breast milk should be refrigerated immediately.
- Within the neonatal unit or ward fresh EBM should be stored in the fridge designated for this purpose at temperatures recorded ≤4°C and used within 48 hours of expressing. If not for use within 48 hours it should be frozen in a freezer at a temperature of at least -18°C.
Freezer
- In some instances breast milk may need to be frozen if the anticipated storage time is more than 24 hours. In these circumstances the breast milk should be frozen as soon as possible after expression to maintain the nutritional and microbiological quality of the milk.
- EBM can be stored for a maximum of 6 months in a freezer with the temperature set to -18°C or below, but preferably less than 3 months as antibody and fat quality will be better.
- At home expressed breast milk should be stored at the back of the fridge and ideally transported to the unit or ward within 24 hour of expressing in cool bag. If there is a delay in visiting the baby then EBM should be frozen and transported as quickly as possible to the neonatal unit in a cool bag with freezer blocks or packed in ice.
The Recent Health Protection Scotland document “Guidance for neonatal units (NNUs) (levels 1, 2 & 3), adult and paediatric intensive care units (ICUs) in Scotland to minimise the risk of Pseudomonas aeruginosa infections from water "3 has indicated that it is no longer acceptable to defrost breast milk using water from a tap.
The following are methods of defrosting EBM which are permitted:
- Defrost in a designated milk fridge.
- Defrost outside the fridge at room temperature. Note, once defrosted and warmed to room temperature milk cannot be returned to the fridge or refrozen. Discard any remaining milk.
- Defrost using a thawing/warming device designed to ensure there is no direct contact with the bottle / syringe with non-sterile water e.g. A Calesca milk warmer from Medela.
- Alternatively, defrost using sterile water which has been warmed in a warming cabinet.
*** DO NOT DEFROST FROZEN BREAST MILK BY PLACING THE CONTAINER IN WARM TAP WATER OR RUNNING UNDER WARM TAP WATER
Additional precautions
- Milk should be used within 12 hours of defrosting
- Label with the date and time that the milk is removed from the freezer and ensure the baby’s name, & CHI number is still present.
- Discard any unused milk.
- Bottles containing EBM should be opened only once and decanted at that time. Feeds of 20mls or less should be drawn up into sterile enteral syringes, capped and identified with baby’s name, unit/CHI number, date and time decanted (to ensure milk is used within an appropriate period). Filled syringes must be returned to the fridge immediately
- Bolus milk feeds may be warmed to body temperature using a Calesca device OR, if no warmer is available, allowed to reach room temperature by removing from the fridge one hour prior to use.
- Breast milk is best used as soon as possible after reaching room/body temperature to prevent bacteria multiplying.
- Where a baby is receiving continuous feeds the syringe should be changed 4 hourly.
- Bolus milk feeds may be warmed to body temperature (37 degrees) using a Calesca milk warmer. Only warm the volume of milk required for a single feed volume. Use the Calesca device to warm the milk to the required temperature and use promptly thereafter. Any residue should be discarded immediately.
- As continuous feeds are administered over 4h they should not be warmed to body temperature but rather administered at room temperature
Maternal Expressed
- All breast milk (mothers own or donor) should be checked by 2 members of staff (or one member of staff and the mother) at the baby’s bedside prior to administering and documented with the 2 checkers signatures. The CHI number and name and date and time expressed should be checked against the baby’s name band.
Donor Milk
- A consent form for the administration of donor milk should be fully completed and inserted into case notes. A photocopy should also be sent to the Donor Milk Bank Coordinator.
- A Donor Milk Record Sheet should be inserted into the notes and used to record all donor milk received using one tear off label from each bottle of milk. The second tear off label must be attached to the batch sheet with the baby’s CHI number and returned to the Milk Bank Coordinator when completed. There is a legal requirement to record end date of all donor milk.
- A record of the donors/recipients of each batch of donor milk is retained indefinitely by the breast milk bank. This should allay any concerns about milk kinship amongst Muslim families. (See Appendix 1) If requested the milk bank will be able to confirm that prospective marriage partners have not received DEBM from their spouse’s mother
- If the error is discovered early, the administered EBM should be aspirated from the stomach
- The nurse in charge and the attending/on call Consultant Neonatologist/Paediatrician should be informed.
- A Datix report should be completed.
- Both the donor mother and the mother/parents of the recipient baby must be informed of the incident by the Consultant, but should not be told each other’s names.
- Details or the error, and the discussion with parent/parents should be documented in the baby’s casenotes.
The major parental concern usually relates to possible transmission of infection. In practice the risk of infection is extremely low. Only HIV, CMV, and HTLV viruses are known to transmit via breast milk (and HTLV is extremely uncommon in our population). No mother who is known to be HIV infected will be expressing and storing milk in the neonatal unit, and there is only a tiny chance that the mother will have seroconverted since she was screened on her booking bloods. CMV commonly transmits via breast milk, although the act of freezing EBM destroys many of the viral particles, which will significantly reduce the risk of infection. Should CMV be contracted in this way, it may cause acute infection, but is very unlikely to have any long term detrimental effects (in contrast to prenatal infection). Infants contracting CMV postnatally are not offered any antiviral medication (except in the rare instance of a significant systemic infection).
The consultant will speak to the parents and apologise for the error in an appropriate manner. The parents should be reassured of the very low risk of infection using the information above. This information may include reassurance that the donor of the incorrect milk was known to be HIV negative at the start of the pregnancy although it is important that the name of the donor is not revealed. This reassurance and apology may be all that the recipient’s parents require.
N.B. This reflects the advice given by the Centre for Disease control (CDC) in the US
Some parents may insist on further reassurance including further virological testing of the donor, (although this will require the consent of the donor).
If the parents insist that they wish further reassurance we would seek consent from the donor to repeat their HIV serology. If, in the unlikely event that this test proved positive there should be consultation with the Infectious diseases team regarding appropriate post-exposure prophylaxis.
We would not routinely offer testing for CMV unless the recipient showed signs or symptoms of acute CMV infection (Note that the incubation period for CMV is 28-60 days).
N.B. If a postnatal infection with CMV were to occur it is more likely that the infection would have originated from the baby’s own mother rather than from a small aliquot of donor milk
We would not routinely test for other infections as they are not reported to transmit via breast milk
|
Time to first expression attempt |
| Number of babies acheiving full milk feeds using maternal EBM alone |
|
Number of mothers acheiving full lactation – only maternal EBM at 4 weeks age |
|
Number of babies receiving breast milk at time of discharge |
|
Episodes of inadvertent administration of the wrong milk |
Resolution on the Use of Donor Human Milk for Muslim Infants [10th January 2016]

Background: The introduction of anonymised donor human milk (DHM) to countries with Muslim populations has been challenged by the Islamic concept of milk kinship. Here the sharing of milk, historically in the form of a wet nurse, creates kinship ties and thus marriage prohibitions between the family of the donor and the recipient. Surveys in the United Kingdom have shown that these beliefs may affect the acceptability of DHM to Muslim parents, and impact on the clinical use of DHM in Neonatal Units in areas with predominantly Muslim populations. Given the many benefits of DHM, especially in the prevention of necrotising enterocolitis in preterm infants, we believed it was necessary to find a resolution to this situation. In order to facilitate this, representatives from the Muslim Council of Britain (MCB), the United Kingdom Association for Milk Banking (UKAMB) , and the British Association of Perinatal Medicine (BAPM) met at a Round Table Discussion on the 26th April 2015 in London.
Aims: To work together in an atmosphere of mutual respect and understanding to give vulnerable infants the best possible start in life, regardless of their religion or ethnicity.
Summary of Round Table Discussion: The National Institute for Health and Care Excellence (NICE) issued guidelines for the use of DHM throughout the United Kingdom in 2010. These state that every aliquot of DHM given must be traceable from donor to recipient. Participants at the Round Table Discussion agreed that this means that, in the future, should there be doubt about whether a potential bride or groom had received DHM from a particular donor, it will be possible to address this.
The process would involve reviewing the recipient’s medical records, in conjunction with records from the relevant Human Milk Bank, to rule out whether they had received milk from the same lactating mother. In the future, electronic barcode tracking is likely to be introduced. This will make the process more straightforward, and also extend the current 30 year limit for the retention of medical records as mandated by NICE.
Resolution: Concerns about milk kinship should not lead to donor human milk being with-held from vulnerable infants, as there are safeguards in place that guarantee the traceability of milk from donor to recipient.
Actions agreed upon:
- To reinforce, via the inclusion of a statement in the soon to be published BAPM Framework for the use of DHM in the United Kingdom, the need for a robust system to ensure the traceability of donated milk. This would ideally be via an electronic bar code system.
- To recommend at the next review of the NICE guidelines on the DHM that records for the use of DHM be kept for longer than the current standard of 30 years.
- To produce a parent information leaflet explaining the rationale for the use of DHM, and steps that can be taken by families who are concerned about the implications of establishment of possible milk kinship.
- To disseminate throughout the United Kingdom, via local religious communities and clinicians in neonatal units in areas with significant Muslim populations.
Signatories:
Dr. Shuja Shafi , Secretary General of the Muslim Council of Britain
Mufti Zubair Butt, Islamic Medical Ethics Advisor to the Muslim Council of Britain
Dr. Syed Mohiuddin, Royal London Hospital
Dr. Morgan Clarke, University of Oxford
Gillian Weaver, UK Association for Milk Banking
Dr. Amanda Ogilvy-Stuart, British Association of Perinatal Medicine
Dr. Thomas Williams, British Association of Perinatal Medicine
- Royal College of Nursing (2013) Breastfeeding in children’s wards and departments (3rd Edition)
- NICE guideline 93 Donor breast milk banks: the operation of donor milk bank services
- Health Protection Scotland (2018) Guidance for neonatal units (NNUs) (levels1,2 & 3), adult and paediatric intensive care units (ICUs) in Scotland to minimise the risk of Pseuddomonas aeruginosa infection from water
- Slutzah M, Codipilly C, Potak D, Clark R and Schanler R (2010) Refrigerator Storage of Expressed Human Milk in the Neonatal Intensive Care Unit. The Journal of Pediatrics, Vol 156, No 1 26 – 28
- Baby Friendly Initiative (2017) BFI standards for neonatal care
Last reviewed: 20 March 2019
Next review: 01 October 2021
Author(s): Fiona Tait – MCN Network Manager – West of Scotland; Patricia Friel – Lead Nurse for Neonatology – GG&C; Andrew Powls – Neonatal Consultant – Princess Royal Maternity; Gillian Bowker – Neonatal Infant Feeding Advisor – GG&C
Approved By: West of Scotland MCN for Neonatology

