RHC Measles guidance
Please see the Health Protection Scotland Quick reference guide for the control of measles incidents and outbreaks in Scotland.
Please also refer to the NHS GG&C Control of Infection Committee Measles guidance. Measles Guidance V7 (interim)
This guidance document will be superseded by an update in the event of widespread community transmission of measles in Scotland.
In 2018 measles virus transmission was re-established in the UK. Measles is the most infectious of all diseases transmitted via the respiratory route, with a reproduction (R) number of between 15 and 20.
The most effective control measure is high uptake of 2 doses of the measles, mumps, and rubella (MMR) vaccine. Vaccination with 1 dose is at least 95% effective in preventing clinical measles, and 92% effective in preventing secondary cases amongst household contacts (1). In the UK, the first dose of MMR vaccine is offered between 12 and 13 months, the second dose at 3 years and 4 months.
Reassuringly vaccine uptake rates in Scotland are high, but still fall below the WHO target of > 95% uptake of 2 doses by 5 years of age. In the quarter ending 30 September 2023, 93.3% of children had had the first dose of MMR vaccine by 24 months of age. This rose to 95.8% for children who had reached age 5 years of age. Uptake of the second dose of MMR vaccine by 5 years of age was 89.6%, rising to 91.0% by age 6 years of age (2).
Whilst the overall risk of measles to the Scottish population is low, current vaccination rates are below the herd immunity threshold, and imported cases could propagate local outbreaks within un- and under-vaccinated communities.
Measles starts with a 2- to 4-day prodromal phase, with high fever, cough, coryza and conjunctivitis, before a maculopapular rash develops.
Fever typically increases during the prodromal phase, peaks (generally >39°C) at around the time of onset of the rash and gradually decreases after that. Measles is very unlikely if there is no history of fever.
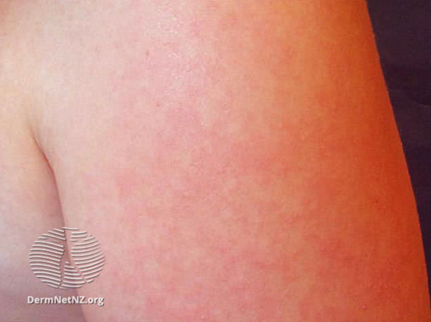
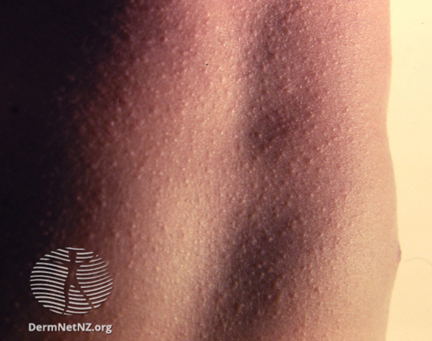
The maculopapular rash generally starts on the face and behind the ears. The number of lesions/spots increases over the first 2-3 days. The rash spreads to the face and trunk, and can become generalised, the lesions can become confluent. The rash is not itchy. It lasts for around 3-7 days, fading gradually. It may end with peeling of the skin. (See pictures below as example).
Koplik spots may appear around the time of the rash, sometimes 1 day before, and last for 2-3 days after the rash appears. These are small white or bluish- white lesions, of about 2-3mm in diameter, on an erythematous base on the buccal mucosa. These can be confused with other lesions in the mouth and therefore their suspected presence is an unreliable marker for measles.
Symptoms typically peak on the first day of the rash.
Clinical course of primary measles infection and its main symptoms
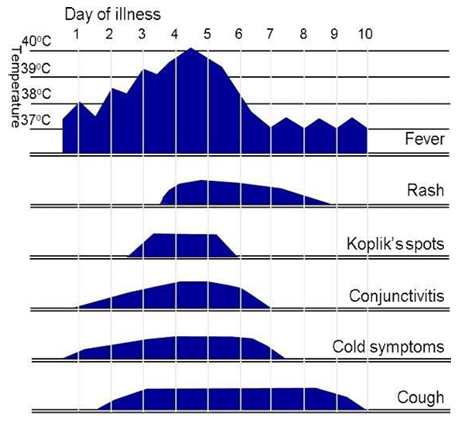
Above figure taken from: UK HAS National measles guidelines, February 2024
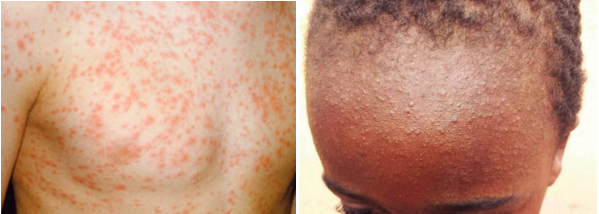
Pictures from UK HAS National measles guidelines, February 2024
Further example pictures available at:
Patients who are immunosuppressed may present with an atypical rash or no rash at all. They may also present directly with pneumonia or encephalitis. Immunosuppressed patients may have prolonged excretion of the virus in respiratory tract secretions and can be contagious for the duration of the illness.
Transmission
Cases are considered infectious from 4 days before to 4 days after onset of the rash.
Significant contact is defined as being in the same room for 15 minutes or more, or face-to-face contact of any length.
For immunosuppressed individuals, any level of contact is assumed significant.
The incubation period is typically around 10 to 12 days but can vary from 7 to 21 days.
Possible complications
Complications are more common and more severe in immunosuppressed patients and young infants. These include:
- Secondary bacterial or viral infections, including pneumonia
- Viral pneumonitis
- Otitis media
- Diarrhoea
- Tracheobronchitis (measles croup)
- Encephalitis (rare – 0.05%-0.1% cases)
- Subacute sclerosing panencephalitis (SSPE) (very rare 0.01% cases), this is a fatal neurodegenerative disorder presenting years after infection.
- Clinicians should be aware that measles remains a rare disease in Scotland and consideration should be given to the differential diagnosis of children with rash and fever, which includes other childhood viral exanthems, Group A Streptococcal infection and Kawasaki disease (appendix 1). In children without fever, other causes of florid rash including erythema multiforme and viral or allergic urticaria should be considered. The wider differential diagnosis should be considered when planning investigation and management.
- Measles should be considered in patients with clinical features as described above, particularly if there is a history of contact with a case of measles, recent travel to an area where measles is circulating (see ‘Update on measles epidemiology in England’ and ‘CDC Global Measles Outbreaks’) or in unvaccinated or partially vaccinated children.
- Membership of a community known to be more susceptible, for example the traveller community, Charedi Orthodox Jewish community, anthroposophical (Steiner) communities, or other local community with low MMR vaccination coverage and attendance at large mass gatherings where substantial mixing occurs between individuals potentially travelling from areas where measles is circulating, are also recognised epidemiological risk factors.
- If testing for measles is being considered, ask the family about any high-risk contacts (immunosuppressed, young infants, pregnant women). Cases are considered infectious from 4 days before to 4 days after the rash onset. Significant contact is defined as being in the same room for 15 minutes or more, or face-to-face contact of any length. For immunosuppressed individuals, any level of contact is assumed significant. Management of high-risk contacts is described below and will be co-ordinated by the Public Health Protection Team.
- All suspected cases should be discussed with the Public Health Protection Team immediately (0141 201 4917 Mon-Fri 9-5pm, 0141 211 3600 and ask for the NHS GG&C Public Health Protection Team on call out of hours) including overnight. The Public Health Protection Team will evaluate the likelihood of measles, and the need for assessment and management of any high-risk contacts.
- If it is agreed that measles testing is required, a throat swab should be sent for measles PCR. This should be an individual standard swab expressed into a viral transport medium (the swab should not be left in the transport medium vial). This should be sent directly from primary care to the West of Scotland Specialist Virology Centre. If the patient presents more than 6 days after onset of the rash, the GP should discuss testing with the West of Scotland Specialist Virology Centre (0141 201 8722). Please ask the GP to email ssvc2@nhs.scot with details of any measles testing. Results should be available the following day, including at the weekend.
- Children and young people with mild or moderate disease and no risk factors for severe complications can be managed in the community with the usual advice on antipyretics and self-care for febrile viral illnesses.
- The possible case should be advised to isolate at home until test results are available. If measles is confirmed, cases should isolate at home for at least 4 days after the rash onset. Parents/carers should be advised about red flag symptoms and signs and when to seek further medical attention. The need for household and other contacts to isolate depends on risk assessment by the Public Health Protection Team.
- Referral to secondary care for assessment should be considered for all children with severe disease, who require supportive care, or who have signs of secondary complications such as encephalitis or pneumonia. As a guide, primary care practitioners should use the same threshold and indicators for referral as they would for other febrile illnesses in children (e.g. need for review/further tests to exclude sepsis/pneumonia, possible need for hospital supportive care such as oxygen or IV fluids).
- Infants under the age of 1 year, children who are immunosuppressed (for definitions see section on ‘Immunosuppressed patients’ below) and pregnant young people with suspected measles should be referred to secondary care for further assessment due to the higher risk of complications in these groups (3).
- Cases of suspected measles should ideally be discussed with the Public Health Protection Team before referral to secondary care, unless the child is severely unwell and requires urgent transfer to hospital. Measles PCR testing can be deferred until the child attends the RHC.
- All referrals from primary to secondary care should be made via the RHC switchboard (0141 201 0000) or Consultant Connect to the GP triage line 9-6pm Monday to Friday, or to the on-call paediatrician/registrar 6pm-9am Monday to Friday and at the weekend (85735) PRIOR TO THE PATIENT BEING SENT. Please confirm that the case has been discussed with the Public Health Protection Team by the Primary Care Team, and whether a throat swab for measles PCR testing has been taken in the community.
- The GP triage paediatrician should list the patient on Trakcare as an ‘ED expect’, contact the CDU team (84672) and the ED Clinical Co-ordinator (84585) to inform of the referral, to ensure that appropriate measures are put in place at the RHC to receive the patient.
Management of possible and confirmed measles presentations in RHC ED
Pre-alert:
Pre-alerted possible or confirmed measles case (Primary Care):
- ED greeter confirms patient expected and contacts ED Clinical Co-ordinator (84585). Patient to wait outside ED entrance whilst transfer arranged.
- ED nurse in charge confirms possible case as per screening questions section below / clarifies definitely confirmed measles case, then identifies appropriate isolation cubicle in CDU patient to be transferred to.
- ED greeter and ED Clinical Co-ordinator should wear appropriate PPE (FFP3 mask, disposable apron and gloves should be used for all routine care. Where there is a risk of splashing of blood/bodily fluids to the face and for aerosol generating procedures, eye protection should also be worn).
- Patient transferred to Room 18 (negative pressure room) in CDU. If this is not available, then patient transferred to Room 20 in CDU. If this is not available, discuss placement with ED Clinical Co-ordinator (84585). Access to CDU via ambulance doors with pathway to CDU room cleared of patients.
- If possible/tolerated & does not compromise their care, the patient should wear a surgical facemask in communal areas.
- Patient triaged and then assessed by the Medical Paediatric Team in appropriate PPE (FFP3 mask, disposable apron and gloves should be used for all routine care. Where there is a risk of splashing of blood/bodily fluids to the face and for aerosol generating procedures, eye protection should also be worn).
- Following assessment, if ongoing concerns regarding possible or confirmed measles, then follow ‘Diagnosis and Management in Secondary Care’ section below.
No pre-alert:
ED greeter identifies ‘possible’ case during ‘RHC ED Greeter ‘Screening’ questions SOP’
- Patient to wait outside ED entrance away from other ED attendees.
- ED Clinical Co-ordinator (84585) contacted and attends front door.
- Confirmation of possible cases and additional ‘screening questions’ conducted by ED nurse in charge (in PPE).
Screening questions:
- A - Does the patient have a high fever AND a rash AND one of the 3 C’s present (Cough, Coryza or Conjunctivitis)?
AND
- B - Has the patient been in face-to-face contact with a CONFIRMED measles case in the past 21 days?
OR
- C - Has the patient been to any area outside of the UK with current measles outbreak or an area of current measles outbreak in the UK in the past 21 days?
Check immunisation status of patient – if had 2 MMR immunisations AND NO confirmed case contact in last 21 days, then no indication to isolate and patient should follow existing patient pathways based on their presenting complaint.
If unvaccinated or partially vaccinated child OR confirmed measles contact in last 21 days then follow below.
- If the patient meets the clinical case definition (A) and has been in face-to-face contact with a CONFIRMED measles case in the past 21 days (B), regardless of vaccine status OR
- If the patient meets the clinical case definition (A) and has travelled to an area of current measles outbreak (C), and is unvaccinated or partially vaccinated, then patient transferred to Room 18 (negative pressure room) in CDU. If this is not available, then patient transferred to Room 20 in CDU. If this is not available, discuss placement with ED Clinical Co-ordinator (84585).
- Access to CDU via ambulance doors with pathway to CDU room cleared of patients.
- If possible/tolerated & does not compromise their care, the patient should wear a surgical facemask in communal areas.
- Patient triaged and then assessed by the ED team in appropriate PPE (FFP3 mask, disposable apron and gloves should be used for all routine care. Where there is a risk of splashing of blood/bodily fluids to the face and for aerosol generating procedures, eye protection should also be worn).
ED clinical assessment of a ‘possible’ case in CDU isolation room
- Clinical assessment of the patient following triage to assess whether likely measles or alternative cause of fever, rash and one of the 3-C's (Cough, Coryza or Conjunctivitis), conducted by ED clinical team. PPE to be worn (FFP3 mask, disposable apron and gloves should be used for all routine care. Where there is a risk of splashing of blood/bodily fluids to the face and for aerosol generating procedures, eye protection should also be worn).
- If suspected measles, then follow ‘Diagnosis and Management in Secondary Care’ section below.
- Clinicians should be aware that measles remains a rare disease in Scotland and consideration should be given to the differential diagnosis of children with rash and fever, which includes other childhood viral exanthems, Group A Streptococcal infection and Kawasaki disease (appendix 1). In children without fever, other causes of florid rash including erythema multiforme and viral or allergic urticaria should be considered. The wider differential diagnosis should be considered when planning investigation and management.
- Measles should be considered in patients with clinical features as described above, particularly if there is a history of contact with a case of measles, recent travel to an area where measles is circulating (see ‘Update on measles epidemiology in England’ and ‘CDC Global Measles Outbreaks’)or in unvaccinated or partially vaccinated children.
- Membership of a community known to be more susceptible, for example the traveller community, Charedi Orthodox Jewish community, anthroposophical (Steiner) communities, or other local community with low MMR vaccination coverage and attendance at large mass gatherings where substantial mixing occurs between individuals potentially travelling from areas where measles is circulating, are also recognised epidemiological risk factors.
- If testing for measles is being considered, ask the family about any high-risk contacts (immunosuppressed, young infants, pregnant women). Cases are considered infectious from 4 days before to 4 days after the rash onset. Significant contact is defined as being in the same room for 15 minutes or more, or face-to-face contact of any length. For immunosuppressed individuals (for definitions see section on ‘Immunosuppressed patients’ below), any level of contact is assumed significant. Management of high-risk contacts is described below and will be co-ordinated by the Public Health Protection Team.
- Unless already notified by the GP before referral to hospital, all suspected cases should be discussed with the Public Health Protection Team immediately (0141 201 4917 Mon-Fri 9-5pm, 0141 211 3600 and ask for the NHS GG&C Public Health Protection Team on call out of hours) including overnight. The Public Health Protection Team will evaluate the likelihood of measles, and the need for assessment and management of any high-risk contacts.
- If it is agreed that measles testing is required, a throat swab should be sent for measles PCR. This should be an individual standard swab in viral transport medium, the swab should not be left in the viral transport medium vial (Trakcare request - ‘Measles/rubella virus PCR’). After 6 days after onset of the rash, discuss testing with the West of Scotland Specialist Virology Centre (0141 201 8722). Email ssvc2@nhs.scot with details of any measles testing. Results should be available the following day, including at the weekend.
- Inform the NHSGG&C Infection Prevention & Control team (86382).
Indications for admission
- There should be a low threshold for admission of infants under the age of 1 years, children who are immunosuppressed and pregnant young people with suspected measles, due to the higher risk of complications in these groups (3).
- Admission should be considered for all children with severe disease, who require supportive care, or who have signs of secondary complications such as encephalitis or pneumonia. In line with IPC guidance patients should be admitted to a negative pressure cubicle until 4 days after onset of the rash. The negative pressure cubicle on ward 2C is cubicle 6, if this is occupied admit to a single cubicle on ward 2C.
- Children and young people with mild or moderate disease and no risk factors for severe complications (high risk - infants under the age of 1 years, children who are immunosuppressed) can be managed in the community with the usual advice on antipyretics and self-care for febrile viral illnesses (see section on ‘Discharge from secondary care’ below).
Treatment
- Management of measles is primarily supportive. There is no specific anti-viral therapy.
- Antibiotics should be given if there is concern about secondary bacterial infection e.g. pneumonia (See the RHC Empirical Antibiotic Therapy in Children guideline).
- IVIG can be considered to attenuate disease in immunosuppressed patients (please discuss with the Paediatric Infectious Diseases Team).
- High dose oral vitamin A may be beneficial in severe measles, but availability is limited in the UK (4). For cases of severe measles discuss with the Paediatric Infectious Diseases Team and pharmacy as to whether this is indicated, on a case-by-case basis.
- Possible cases should be advised to isolate at home until measles test results are available.
- Possible and confirmed cases should be advised to avoid contact with vulnerable people (e.g. young infants, pregnant and immunosuppressed people) where possible.
- Confirmed measles cases should remain at home for at least 4 days after the rash onset, ideally until fully recovered.
- The need for household and other contacts to isolate depends on risk assessment by the Public Health Protection Team. The Public Health Protection Team will provide this advice to families.
- Parents/carers should be advised about red flag symptoms and signs and when to seek further medical attention.
The following recommendations are based on the Health Protection Scotland Guidance for the Control of Measles Incidents and Outbreaks in Scotland, revised May 2019.
Young infants, pregnant young people, and immunosuppressed children and young people may benefit from post-exposure prophylaxis. This is with immunoglobulin or the MMR vaccine.
The recommendation to give post-exposure prophylaxis should be made by the Public Health Protection Team, following a risk assessment.
MMR vaccine will be administered in the community.
Immunoglobulin will be administered at the RHC. The preparation and dose of immunoglobulin varies according to the indication. If there are queries about the administration of immunoglobulin, discuss with the on-call pharmacist and the Paediatric Infectious Diseases Team.
Infants under 12 months of age
Infants under 12 months of age are unvaccinated and at high risk of developing measles if exposed. Recommended post-exposure prophylaxis depends on their age and whether they are a household contact and includes both Subcutaneous Immunoglobulin (SCIG), referred to as Human Normal Immunoglobulin (HNIG), and MMR vaccination. See the table below for guidance.
Post-exposure prophylaxis in infants of UK born mothers*
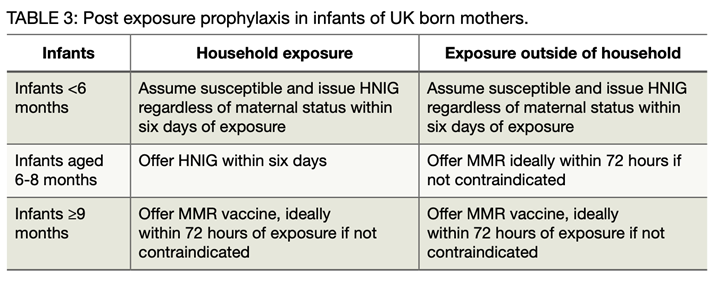
*As the pattern of maternal antibody waning in infants shows significant geographical variation and as vaccination programmes were introduced at different times, this advice may not be applicable to infants of mothers born outside the UK. In such cases an individual risk assessment is required by the Public Health Team.
Guidance for the Control of Measles Incidents and Outbreaks in Scotland, Scottish Health Protection Network, Health Protection Scotland, May 2019
It is the responsibility of the Public Health Protection Team to contact the parent/carer and discuss the recommendations. The discussion will be documented on the patient’s Clinical Portal record.
If the recommendation is for MMR, this will be arranged in the community.
If the recommendation is for immunoglobulin, the Public Health Protection Team will discuss the case with the on call Paediatric Infectious Diseases Team (Consultant via the RHC switchboard 0141 2010000). The Paediatric Infectious Diseases Team will then contact the RHC Clinical co-ordinator (85770) to determine when and where the patient can attend. The location may need to be determined on a case-by-case basis, will depend on capacity and nursing resource. The Paediatric Infectious Diseases Team will then contact the patient with details of when and where to attend.
Patients should be booked in on arrival to the RHC ED, admitted under the care of the Paediatric Infectious Diseases Team.
Please note that contacts are not considered infectious until 5 days after exposure, so standard infection prevention and control measures are sufficient until this time.
All patients should have a set of observations and weight on arrival. The Paediatric Infectious Diseases Team should be contacted to discuss consent for and prescribe the immunoglobulin.
Patients should be observed with a set of observations 30 minutes after administration of immunoglobulin, prior to discharge home.
Treatment with Subcutaneous Human normal immunoglobulin (HNIG) for infants under 6 month of age, or household contacts under 9 months of age
Please complete the immunoglobulin request form, using the red indication ‘specific antibody deficiency’ and give to the 2C pharmacy team (office on ward 2C). Approval can be granted retrospectively if out of hours.
The product supplied will depend on availability, in March 2024 the preferred local products are Hizentra, followed by Cutaquig. Other products may also be used if the preferred products are not available, as discussed with the pharmacy team.
Dose: 0.6ml/kg to a maximum of 1g
Administered subcutaneously or intramuscularly. Please note the intramuscular route is off label, but is suggested as an alternative practical route of administration in this situation by the UKHSA, given the clinical imperative to treat these contacts urgently. The intramuscular route of administration has been agreed as the preferred route at the RHC.
For intramuscular administration The Green Book recommends that if volumes of more than 3ml are needed, immunoglobulin should be given in divided amounts and given into different sites.
Subcutaneous administration should only be done by nursing staff with competence, with the appropriate infusion device/pump. See product Summary of Product characteristics (SPC) for subcutaneous administration instructions.
Please discuss with the ward/on-call pharmacist if needing further advice.
Immunosuppressed patients
Immunosuppressed patients also require a risk assessment and may require measles post-exposure prophylaxis, as intravenous immunoglobulin (IVIG).
The recommendation to give post-exposure prophylaxis should be made by the Public Health Protection Team, following a risk assessment.
If any immunosuppressed person (e.g. patients with leukaemia or on high dose immunosuppressant) is exposed there is a very low threshold for follow-up: even a very short exposure (minutes) should trigger investigation. In a highly immunosuppressed child or young person who is unlikely to be immune, it may be worth considering prophylaxis where the possibility of exposure has occurred e.g. by entering a room within a short period after a case has been present.
All immunosuppressed individuals should be considered for treatment with IVIG as soon as possible after the exposure occurred (preferably within 3 days, but treatment may be effective within 6 days).
People with severe defects of cell mediated immunity who are on regular IVIG replacement therapy do not require additional IVIG if the most recent dose was administered ≤3 weeks before exposure.
See for definitions of immunosuppressed individuals, and further details on risk assessment.
If a specialty team is contacted by a parent/carer with concerns about possible exposure, this can be discussed with the Public Health Protection Team (0141 201 4917 Mon-Fri 9-5pm).
Following exposure to a confirmed case of measles, it is the responsibility of the Public Health Protection Team to contact the parent/carer and discuss the recommendations. The discussion will be documented on the patient’s Clinical Portal record.
Following risk assessment, the Public Health Protection Team should contact the on call responsible clinical team for the individual, to discuss the recommendations (Consultant via the RHC switchboard 0141 2010000).
If measles IgG testing or IVIG is indicated, the responsible clinical team should liaise with the Clinical co-ordinator (85770) to determine when and where the patient can attend. The location may need to be determined on a case-by-case basis, will depend on capacity and nursing resource. The responsible clinical team should then liaise with the patient to arrange admission.
Please note that contacts are not considered infectious until 5 days after exposure, so standard infection prevention and control measures are sufficient until this time
Patients should be booked in on arrival to the RHC ED before being transferred to the allocated clinical area, admitted under the care of the responsible clinical team.
If attending for IVIG, patients should have a set of observations and weight on arrival. The responsible clinical team should be contacted to review, should ensure IV access, discuss consent and prescribe the immunoglobulin.
Patients should be observed, with a set of observations 30 minutes after administration, prior to discharge home.
Treatment with Intravenous Immunoglobulin (IVIG) for immunosuppressed children
Please complete the immunoglobulin request form [Sharepoint link], using the red indication ‘specific antibody deficiency’ and give to the 2C pharmacy team (office on ward 2C). Approval can be granted retrospectively if out of hours.
The preparation used will depend on available stock. There is a likelihood that immunosuppressed patients will have been treated with IVIG in the past. If the preparation is known, use the same brand if available.
Dose - 0.15 g/kg.
For administration guidance refer to the product Summary of Product characteristics (SPC) or ‘Normal Immunoglobulin IV schedules – other brands’ [Sharepoint link], available on the Staffnet hub.
Please prescribe IVIG on both the ‘Normal Immunoglobulin IV schedules – other brands’ form and also on HEPMA.
Please note that patients with known severe defects of cell-mediated immunity, who are on regular IVIG replacement therapy, do not require additional IVIG if the most recent dose was administered 3 weeks or less before exposure.
Please discuss with the ward/on-call pharmacist if needing further advice.
If unsure about recommendations for testing or prophylaxis, please discuss with the Paediatric Infectious Diseases Team.
Detailed NHSGG&C guidance is available here.
Non-essential visitors should be minimised.
Parents, carers or visitors with symptoms should not be permitted to enter a care area, unless it is considered essential.
PPE is not required for household contacts.
Healthcare workers are encouraged to know their own measles vaccine and/or serology status. Those unsure of their status should contact the Occupational Health Team (directly by email occupational.health@ggc.scot.nhs.uk or telephone 0141 201 0600 or through their line manager).
Healthcare workers who are not immunosuppressed and have had at least 2 doses of a measles-containing vaccine, or who have documented positive measles serology (at any time) are considered immune. For these individuals no action is required other than to be alert for the symptoms of measles in the 21 days following exposure due to the rare possibility of breakthrough infection.
Healthcare workers who are non-immune or unsure of their status, or those who are pregnant or immunosuppressed, should contact the Occupational Health Team on 0141 202 0594 or 0141 201 0600 for advice. They may be offered post-exposure MMR vaccination (or IVIG prophylaxis if pregnant/immunosuppressed) and urgent serology testing.
They can continue to work until 5 days after exposure. Those with positive serology (immune) can return to work, those with negative serology (non-immune) and confirmed exposure will be excluded from work until 21 days post-exposure. Exclusion time may be reduced if post exposure vaccination is undertaken - this should be confirmed with the Occupational Health Team on 0141 202 0594 or 0141 201 0600.
Roseola (exanthema subitem, sixth disease)
- Huma herpes virus 6 (HHV6), occasionally HHV7.
- Most infections in infants 6 to 24 months of age. By 2 years of age 90% children are immune to HHV6.
- Transmission via the respiratory route/droplet.
- Incubation period 5 to 15 days.
- Presents with 3-5 days of fever, coryza, a maculopapular rash appears on the face and body as the fever is resolving and the patient is otherwise improving.
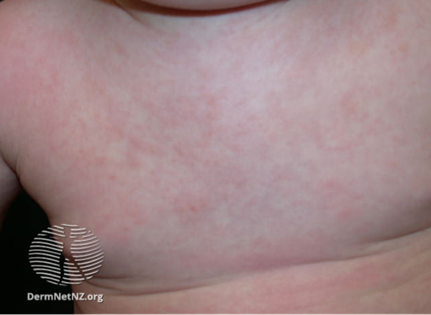
https://dermnetnz.org/topics/roseola
Fifth disease (‘slapped cheek syndrome’)
- Parvovirus B19.
- Children of all ages, young adults.
- Transmission via the respiratory route/droplet.
- Incubation period 13 to 18 days.
- Presents prodromal symptoms of fever, coryza, headache, ‘slapped cheeks’ followed by a rash (evanescent lacy-pattern erythema), mostly on the extremities.
https://dermnetnz.org/cme/viral-infections/specific-viral-exanthems
Rubella (German measles)
- Rubella virus.
- Rubella is prevented by the MMR vaccine, so few cases are now reported.
- Transmission – respiratory route/droplet.
- Incubation period 12 to 21 days.
- Asymptomatic in 50%. Prodrome malaise, cough, coryza, sore throat, with or without fever, post-auricular and sub-occipital lymphadenopathy. Rash is non-specific, ‘pale pink’, generally mild, most often on the face and behind the ears, where it starts before spreading. Young people may develop arthralgia or arthritis.
Scarlet fever
- Group A Streptococcus.
- Pre-school and school-aged children.
- Transmission respiratory route/droplet.
- Incubation period 1 to 5 days.
- Presents with sore throat, pharyngeal exudate, high fever. Maculopapular rash appears 12 to 48hrs after the start of symptoms. Starts on the abdomen, spreading to the neck, back and limbs. A white coating of the tongue may be present (‘strawberry tongue’).
- UK Health Security Agency. National measles guidelines January 2024 [Internet]. 2024 [cited 2024 Jan 21].
- Childhood immunisation statistics Scotland - Quarter ending 30 September 2023 - Childhood immunisation statistics Scotland - Publications - Public Health ScotlandChildhood immunisation statistics Scotland - Quarter ending 30 September 2023 - Childhood immunisation statistics Scotland - Publications - Public Health Scotland.
- National Institute for Health and Care Excellence (January 2024). Scenario: Management of measles. Scenario: Management | Management | Measles | CKS | NICE
- National Foundation for Infectious Diseases. Vitamin A for the Management of Measles in the US. March 2020. Website last accessed 17/2/2024. Vitamin A for the Management of Measles in the US – NFID
Last reviewed: 09 October 2024
Next review: 30 April 2026
Author(s): Dr Katherine Longbottom, Consultant General Paediatrics & Paediatric Infectious Diseases, RHCG, Dr Louisa Pollock, Consultant General Paediatrics & Paediatric Infectious Diseases, RHCG, Dr Steve Foster, Consultant in Paediatric Emergency Medicine, RHCG, Dr Ciara Carrick, Consultant in Paediatric Emergency Medicine, RHCG.
Author Email(s): steven.foster@ggc.scot.nhs.uk
Co-Author(s): Ms Shahad Abbas, Advanced Pharmacist - Antimicrobials and Medical Paediatrics, RHCG.
Approved By: RHC Medical Paediatrics & Paediatric Emergency Department Clinical Governance Groups

