Mucositis - diagnosis and treatment
The purpose of this document is to provide guidance for the management of mucositis in children and young people with cancer receiving chemotherapy/radiotherapy.
Oral complications occur commonly during and/or following cancer treatment, particularly in patients undergoing haemopoietic stem cell transplant (HSCT). This can result in pain, difficulty in swallowing, phonation and poor nutrition, severely impacting on the patient’s quality of life. Mucositis, painful inflammation and ulceration of the mucous membranes, is one of the commonest side effects of chemotherapy. The oral mucosa consists of rapidly dividing cells that are especially susceptible to the damaging effects of cytotoxic therapy. Oral complications during chemotherapy and radiotherapy can arise not only from direct injury to the oral mucosa, but secondary to cytotoxic induced myelosuppression resulting in profound neutropenia. Good oral hygiene is crucial as without it, mucositis can lead to secondary bacterial, fungal and viral infections. Minimising mucositis is crucial to the prevention of these complications and to promoting good quality of life.
Mouth Care Guidelines for Parents, Carers & Children (available via the Dentist).
Medical, nursing, pharmacy and dental staff.
- Apron
- Otoscope / torch
- Sick bowl
- Soft toothbrush
- Swabs (if required)
- Toxicity score / treatment table (OAG scoring, see Appendix 2)
- Pain assessment tool
Diagnosis
- The patient’s mouth should be regularly examined by a nurse/doctor/dentist using the otoscope/torch as part of routine assessment to observe any changes ie inflammation, ulcerated areas and candida plaques using the scoring chart below (see Appendix 1). External changes ie sore or vesicles on the lips should also be noted and bacterial/viral swabs taken where necessary.
- All findings (ie affected area, grading, pain scale) should be documented in the casenotes.
- Swabs should be sent for microscopy and bacterial and viral culture as appropriate.
Treatment
- Patient should be encouraged to maintain good oral hygiene and to maintain an adequate volume of oral fluid.
- A mouthwash appropriate to the grade of mucositis (see Appendix 3) should be prescribed.
- Appropriate pain control is recommended together with the continuation of good oral hygiene, as tolerated. The patient's pain should be assessed using the assessment tool in Appendix 3.
- Patients should be referred for laser therapy if thought to be of benefit.
For further information contact:
Consultant in charge of the child or the Dentist attached to the unit
|
DENTAL CARE / TREATMENT |
||
| At Diagnosis: Oral & dental assessment |
Oral hygiene advice should be given to children and parents prior to commencing cancer treatment and this should be provided both verbally and in writing.
|
|
|
During cancer treatment: |
|
|
|
Post Treatment: |
Parents should be informed of the possible long term dental or orofacial effects of treatment. Monitored for these effects regularly By usual dental provider with clear communication and guidance from the cancer centre |
|
|
BASIC ORAL CARE |
||
|
At Diagnosis & During Treatment: |
|
|
|
ORAL COMPLICATIONS (FOR DOSES SEE BNF FOR CHILDREN) |
||
| Prevention | Treatment | |
|
Mucositis |
|
|
|
Pain |
|
|
|
Candidiasis |
|
|
|
Xerostoma |
|
|
|
Herpes |
|
|
Routine recommended oral care
| All Patients | HSCT / PBSCI Patients |
| Oral Assessment | Oral Assessment |
|
Baseline assessment on admission but daily OAG* not required unless:
Then daily OAG until discharge |
Baseline assessment on admission & daily until discharge |
| Mouth Care | Mouth Care |
|
Brush teeth and gums well twice daily with a fluoride toothpaste and soft tooth brush. + Daily fluoride supplements If oral candida develops treat with short courses of oral fluconazole If herpes simplex develops treat with aciclovir |
Brush teeth and gums well twice daily with a fluoride toothpaste and soft tooth brush. + Daily fluoride supplements + Ambisome 1mg/kg THREE times weekly (Mon/Wed/Fri) NB: 2mg/kg for HSCT patients + Aciclovir prophylaxis as per unit policy |
| Category | Method of Assessment | 1 | 2 | 3 |
|
Swallow |
Ask the child to swallow or observe the swallowing process. Ask the parent if there are any notable changes. |
Normal. |
Difficulty in swallowing |
Unable to swallow at all. |
|
Lips and corner of mouth |
Observe appearance of tissue |
Normal. |
Dry, cracked or swollen |
Ulcerated or bleeding |
|
Tongue |
Observe the appearance of the tongue using a pen-torch to illuminate the oral cavity |
Normal. |
Coated or loss of papillae with a shiny appearance with or without redness and/or oral Candida |
Ulcerated, sloughing or cracked |
|
Saliva |
Observe consistency and quantity of saliva |
Normal. |
Excess amount of saliva, drooling |
Thick, ropy or absent |
|
Mucous membrane |
Observe the appearance of tissue using a pen-torch to illuminate the oral cavity |
Normal. |
Reddened or coated without ulceration and/or oral Candida |
Ulceration and sloughing, with or without bleeding |
|
Gingiva |
Observe the appearance of tissue using a pen-torch to illuminate the oral cavity |
Normal. |
Oedematous with or without redness, smooth |
Spontaneous bleeding |
|
Teeth |
Observe the appearance of teeth using a pen-torch to illuminate the oral cavity |
Normal. |
Plaque or debris in localised areas |
Plaque or debris generalised along gum line |
|
Voice |
Talk and listen to the child. Ask the parent if there are any notable changes. |
Normal tone and quality when talking or crying |
Deeper or raspy |
Difficult to talk, cry or not talking at all |
Oral assessment guide, 2004 - Adapted from Eilers, J. Berger, A. and Peterson, M. (1988) by GOSH Oral Care Working Party. © GOSH
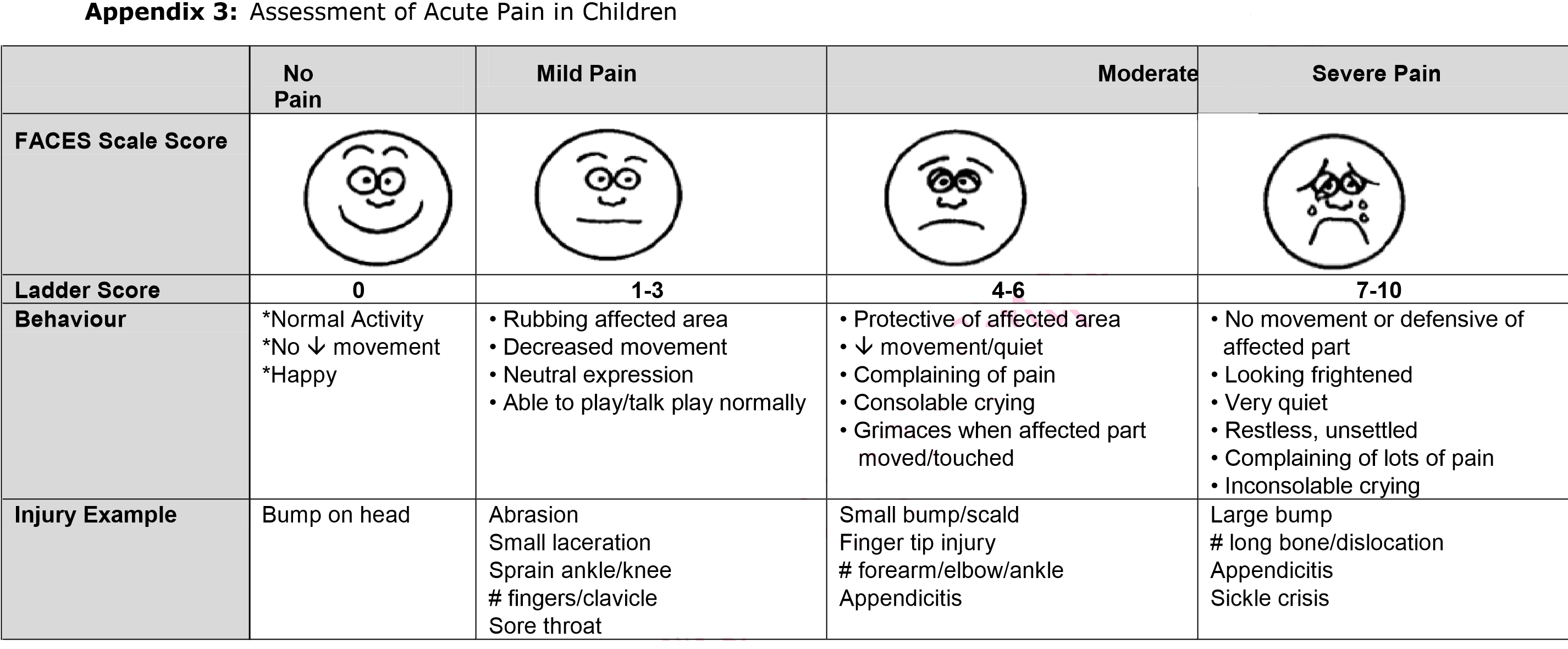
|
Recommendations for the Treatment of Oral Mucositis |
|
|
|
|
|
Recommendations for the prevention of oral mucositis |
|
|
|
Drug |
Dosage |
Comments |
|
Aciclovir:
|
Herpes simplex prophylaxis in immunocompromised: 1month-2years: 200mg four times a day |
Topical preparation is useful if applied to early onset of cold sores, which often reactivate during chemotherapy but should only be used with systemic treatment. |
|
Ambisome: IV infusion |
Prophylaxis: 2mg/kg THREE times weekly (Mon/Wed/Fri) |
Prophylactic antifungal dose. Infuse over 1 hour |
|
Difflam oral spray
|
Difflam Spray: <6years: 1 spray per 4kg of body weight (max 4 sprays at any one time) every 1.5-3hrs |
Due to the anaesthetizing effect on the pharynx, difflam should not be used for pre-school children or immediately before meals as it could lead to choking. Care should be taken with very hot or cold drinks. |
|
Fluconazole:
|
Mucosal candidiasis (except genital): 1 month-12 years: 3-6mg/kg (max 200mg) on the first day then 3mg/kg (max 100mg daily) for 7-14 days in oropharangeal candidiasis (max 14 days except in severely immunocompromised patients) 12-18 years: 50mg daily (max 100mg daily) for 7-14 days in oropharyngeal candidiasis (max 14 days except in severely immunocompromised patients) |
Fluconazole should only be used to treat proven fungal infections. Fluconazole should NOT be used prophylactically except in Neuroblastoma high risk patients. Monitor LFTs fortnightly, discontinue if signs and symptoms of liver disease. Intravenous Fluconazole can be used if the patient unable to tolerate oral medication. Consider interaction with chemotherapy when Fluconazole is used. |
|
Gelclair:
|
Mix entire sachet with 40ml of water Discard any unused sachet after 24 hours |
Gel which provides pain relief by adhering to the mucosal surface of the mouth Gelclair can be used undiluted Do not eat or drink for at least 1 hour following treatment |
|
Morphine:
|
See "Guidelines for the Management of Acute and Post-Operative Pain". Please note that these guidelines only provide starting doses for oral immediate release morphine and not MST preparations Consult with RHC Pain Team See BNF for Children for doses |
If using part of a MST sachet mix well with water and use immediately. MST sachets should not be used for doses <5mg as sachets do not disperse equally therefore dosing may be inaccurate |
|
Saliva stimulant |
Consult Pharmacy for available preparations |
|
|
Teething gels: |
Consult Pharmacy for available preparations |
Calgel |
|
Caphosol |
Mix blue ampoule (A) with clear ampoule (B) Use four times a day |
Rinse half of solution around mouth for 1 min then spit out. Repeat with remaining solution. |
|
Chlorhexidine gluconate 0.2% mouthwash |
Rinse 5-10ml (depending on patient size) around mouth for about 1 min four times a day |
Can apply to mucosa on swabs for very young patients |
Mouthcare for Children & Young People with Cancer. Children's Cancer & Leukaemia Group (CCLG) Evidence Based Guidelines. Version 1.0 February 2006.
BNF for Children
EUSApharm Medical Information at: www.caphosol.com
Gelclair Product Information Leaflet. Produced by Alliance Pharmaceuticals.
Last reviewed: 28 August 2023
Next review: 31 August 2026
Author(s): J Sastry
Version: 4
Approved By: Schiehallion Clinical Governance Group
Document Id: RHC-HAEM-ONC-029

