Varicella zoster infection (chickenpox): management in children
exp date isn't null, but text field is
Objectives
To provide guidance on the management of chickenpox and its complications in children under the age of 16, and the use of post exposure prophylaxis in neonates and those at risk of severe infection. This guideline does not cover the treatment of young people who are pregnant and additional advice should be sought from obstetrics.
Chickenpox is an infection caused by the varicella zoster virus which is characterised by a vesicular rash. In most children its course is benign but, in some cases, it can be severe and result in significant complications. The infectious period lasts from 24 hours before the onset of the rash until all the vesicular lesions are crusted. Neonates, immunocompromised children and pregnant woman are at the highest risk of complications however severe complications can be seen in previously healthy children.
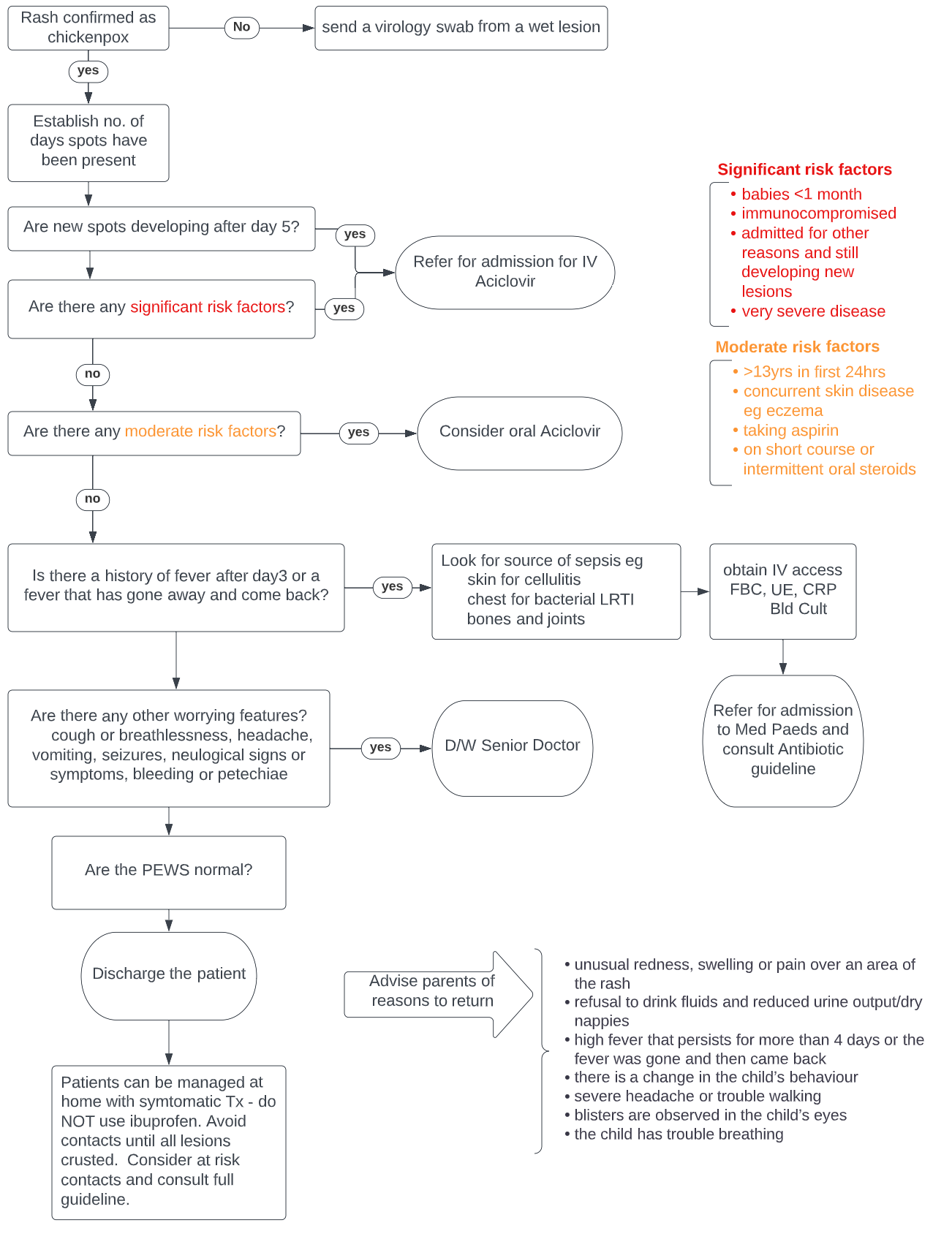
- Fever - can precede the rash by 1-2 days and usually subsides by day 3 of the rash
- Rash - generally starts centrally (head and trunk) as red macules and progresses through the papular and vesicular stage before crusting. New lesions tend to appear over the first 5 days. Lesions are typically in different stages, intensely itchy and may affect any area of the body, including the pharynx and tonsils.
In most cases diagnosis is based on clinical symptoms, and no further investigations are required. In cases of doubt send a skin swab of a wet skin lesion to virology for PCR.
The following patients are at risk of developing severe infection and require admission:
- Babies under 1 month of age
- Immunocompromised children (see appendix 1)
- Children still developing new lesions after day 5 - risk of disseminated disease
- Children with serious complications (bacterial secondary infection, neurological or respiratory complications) – see section 6
In immunocompetent children over 1 month of age who are otherwise healthy, anti-viral treatment is not normally required.
For the following children who are at risk of moderate disease5, consider oral Aciclovir for FIVE days - dose as per BNF-c
- Over 13 years of age if presenting within the first 24 hours
- Concurrent severe skin disease e.g. eczema
- Underlying pulmonary disease e.g. Cystic Fibrosis, chronic lung disease, asthma requiring inhaled steroids, interstitial lung disease
- Children taking aspirin
- Those currently receiving short-course or intermittent oral corticosteroids e.g. 1-2mg/kg prednisolone for viral induced wheeze or exacerbation of asthma
It is important to advise families on ensuring good fluid intake whilst taking aciclovir.
In the following patients, IV aciclovir should be commenced – dose as per BNF-c or for neonates please use the West of Scotland Neonatal drug monographs
- Babies under 1 month: 7 days of treatment
Those infants who are well, have low number of lesions and whose mother has a history of chicken pox may not require a full 7 days of treatment. Discussion with ID should be had in these cases after 48 hours of treatment. - Immunocompromised Children: 7 days of treatment or until no new lesions for 48 hours (the higher BNF immunocompromised dosage should be used)
- Children still developing new lesions for more than 5 days: Treat until 48 hours after the appearance of the last lesions with the higher BNF immunocompromised dosage.
- Children admitted with either virally mediated complications, or serious bacterial complications with continuing new lesions - see section 7 for further detail
- Consider IV aciclovir for severe varicella in children not previously known to be immunodeficient as this can be an indicator infection for severe immune compromise
Aciclovir is renally excreted and had been shown to cause renal impairment, particularly in dehydrated patients. Adequate hydration should be maintained and there should be a low threshold for considering IV fluids. Accurate fluid balance should be recorded. U&Es with creatinine should be monitored every 2-3 days, or daily in patients with underlying renal dysfunction. For further information and dosing in renal impairment, refer to the SmPC, sections 4.2 and 4.8.
Symptomatic treatment for all Children:
- Paracetamol for fever
- Topical calamine can be used but there is no evidence basis, and it can be felt to dry the skin and increase itching
- Antihistamines (e.g chlorphenamine) can be used for itch
- Parents should be advised to keep children’s nails short, cover affected skin with loose light clothing and encourage oral fluids
NSAIDS (e.g Ibuprofen) should be avoided.
The following are red flags for severe disease or complications. In any child presenting with a red flag symptom or sign, discussion with the paediatric medical registrar or ED/medical consultant should be considered prior to discharge.
- Recurrence of fever
- Respiratory symptoms
- Persistent fever beyond day 3 of initial rash
- Neurological signs and symptoms
- Bleeding or petechiae
- Haemorrhagic rash
- Producing new lesions for greater than 5 days
Secondary bacterial Infection
This is the most common complication
There needs to be a high index of suspicion for secondary bacterial infection if there is persisting fever beyond day 3 or recurring fever. In all children, fully expose and look for evidence of skin infection.
Children are at risk of invasive Group A Streptococcal infection and Staphylococcus aureus infection which can present as sepsis, cellulitis, pneumonia, septic arthritis, and osteomyelitis.
Treat as per GGC guideline for Empirical antibiotic therapy in children NOTE: For the systemically unwell child with varicella and signs of secondary bacterial infection the antibiotic treatment regime should include high dose clindamycin –dose as per BNF-c
Necrotising Fasciitis is a rare complication associated with varicella.
Consider in a child with fever, tachycardia, swelling and severe pain (can be present without fever). Urgent surgical review is required, and antibiotics should be discussed with microbiology/Infectious diseases (ID).
Toxic Shock syndrome can also be associated with varicella.
It is characterised by fever, hypotension, rash, multi-organ involvement and desquamation.
Treatment involves fluid resuscitation and IV antibiotics - high dose cefotaxime and clindamycin – dose as per BNF-c . PICU review should be requested. IV immunoglobulin can be considered after discussion with the ID consultant on call. This is a blue/group 4 indication under the national demand programme. An IVIg request form MUST be completed and sent to Pharmacy. Out of hours stocks can be obtained from the EDC (ground floor, QEUH) or PICU and the request form completed and submitted the following working day.
Varicella Pneumonia
This is more common in adolescents and adults
Patients presents 1-6 days after the onset of the rash with cough, dyspnoea, fever and hypoxia with fine crackles over the lung fields. Haemoptysis can be present.
CXR shows diffuse bilateral nodular infiltrates
Varicella pneumonia is treated with IV aciclovir - dose as per BNF-c but be aware that secondary bacterial pneumonia (especially Group A Streptococcus) is more common in children. Concomitant antibiotics may be required.
Varicella Encephalitis
This can present with headache, vomiting, fever, confusion or seizures around 2-6 days after the onset of the rash.
The need for imaging should be discussed with on call general paediatric consultant.
LP shows pleocytosis with lymphocyte predominance
Treat with high dose IV aciclovir - CNS dose as per BNF-c and cover with cefotaxime CNS dose as per BNF-c pending LP results.
Cerebellar Ataxia
This is the most common neurological complication. Patients can present at around 1 week with a developing broad-based gait. Other symptoms can include irritability, vomiting, tremor, headache, slurred speech, hypotonia and nystagmus.
Children usually have a complete recovery in 2-4 weeks
Some cases of varicella cerebellar ataxia can include cerebellitis. A rare complication can be acute hydrocephalus secondary to fourth ventricle obstruction. Clinical assessment should look for signs of raised intracranial pressure. All cases should be discussed with neurology. Overnight children should be admitted under the general paediatric team and discussed with neurology in the morning unless there are concerns regarding raised intracranial pressure in which case urgent out of hours CT scan should be arranged.
Continuing Lesions post day 5
Severe varicella is associated with prolonged viral replication. It is often associated with fever or other organ involvement. It can be a sign of immune compromise.
All children who are unwell with continuing new lesions after day 5 should be admitted for IV aciclovir -Immunocompromised dose as per BNF-c even if no previous evidence of immune compromise.
Involvement of the eye
Where lesions develop that affect the eye an ophthalmology consult should be sought.
Hepatitis
Mild subclinical hepatitis is common. Liver enzymes are increased but recover without treatment. Rarely, mainly in immunocompromised patients, a symptomatic hepatitis can occur and is associated with a coagulopathy. No dose adjustment of aciclovir is necessary.
Haemorrhagic lesions/Petechiae
Haemorrhagic varicella is more likely to occur in immunocompromised children.
Thrombocytopenia can occur but is generally asymptomatic and recovers without treatment
In cases of petechiae check FBC, coagulation and LFTS and in cases of haemorrhagic varicella commence IV aciclovir- Immunocompromised dose as per BNF-c.
Where there are bacterial complications; if there is evidence of ongoing viral replication (e.g. new lesions) add in IV aciclovir, as per section 4.
Advise parents to bring their children back to hospital if they develop the following complications:
- unusual redness, swelling or pain over an area of the rash
- refusal to drink fluids and reduced urine output/dry nappies
- high fever that persists for more than 3 days after the rash or the fever was gone and then came back
- there is a change in the child’s behaviour
- severe headache or trouble walking
- blisters are observed in the child’s eyes
- the child has trouble breathing
The aim of post exposure prophylaxis is to protect individuals who are at high risk of severe chickenpox, are varicella IgG negative and have:
- significant exposure to chickenpox or shingles during the infectious period - see appendix 1
AND
- a condition which increases the risk of severe chickenpox – see appendix 1
If a high-risk child of unknown antibody status is exposed to chickenpox, then antibody status should be checked urgently. Where the child has no known history of chicken pox infection, prophylaxis should be given from day 7 to day 14 post exposure*. If there is a history suggestive of previous varicella infection, then prophylaxis should be given once a negative antibody result is obtained (VZV IgG <150mIU/ml). This should be discussed with the medical team in charge of the child’s overall care.
Where a patient presents after day 7 of exposure, a 7 day course of antivirals can be started as soon as possible and up to day 14 post exposure, if necessary.
*The day of exposure is defined as the date of onset of the rash if the index case is a household contact OR the date of first or only contact if the exposure is on multiple or single occasion(s) respectively.
Patients receiving regular intravenous or subcutaneous immunoglobulin do not require further prophylaxis.
If in doubt whether a child should receive post exposure prophylaxis contact their relevant medical team. Rheumatology have separate guidance and should be contacted directly for advice.
For further information access this link and see appendix 1 UKHSA: Guidelines on post exposure prophylaxis for varicella/shingles (April 2022)
Both Varicella immunoglobulin (VZIG) and oral aciclovir are suitable for prophylaxis. However, The UK Health Security Agency (formerly Public Health England) and Public Health Scotland now recommends that most patients (excluding neonates exposed within 7 days of delivery) should be managed with high dose oral aciclovir for 7 days starting on day 7 post exposure (see definition above). Where a patient presents after day 7 of exposure, a 7 day course of antivirals can be started as soon as possible and up to day 14 post exposure, if necessary. Dose as per BNFc.
Patients may still get chickenpox following prophylactic treatment and should be advised on the signs of infection and to present immediately should they develop symptoms.
If there are concerns about malabsorption or where oral antivirals are contraindicated then VZIG should be considered and be given as soon as possible, within 7 days of exposure. For patients presenting after 7 days there may still be benefit in giving VZIG up to 10 days post exposurel2. Varicella antibodies should be checked and VZIG only given if negative or result <150mIU/ml. VZIG dosing recommendations can be found in the UKHSA Guidelines on post exposure prophylaxis for varicella/shingles (April 2022, page 10)
If a patient represents following a second exposure, they should be reassessed, and blood taken for varicella IgG. If they have not seroconverted they will require a further course of aciclovir (including patients who have just completed a course of antivirals), given in the same way starting 7 days after the subsequent exposure. Patients who have recently received VZIG and have a second exposure may need a further dose if the first dose was given more than 3 weeks ago. If the exposure was significant, a further dose should be given if within 3-6 weeks following the initial administration of VZIG without further testing. If more than 6 weeks following VZIG, antibody levels should be rechecked and a decision to treat made following results.
At highest risk of severe disease are infants whose mothers develop chicken pox (not shingles) 7 days before to 7 days after delivery.
In these babies IM VZIG should be given as soon as possible and within 7 days of birth or within 7 days of onset of the disease in the mother if this is longer.
In addition, in the highest risk infants, whose mother developed varicella between 4 days prior to delivery and 48 hours post-delivery, consider admission for 5 days IV aciclovir prophylaxis - dose as per BNF-c. This should be commenced on day 7 of maternal varicella2,7.
Post exposure prophylaxis is also recommended for:
- Infants of non-immune mothers (Varicella IgG <150mIU/ml) who have a significant non maternal exposure within the first 7 days of life. If the mother has no or uncertain history of chicken pox or has received the varicella vaccine, urgent antibody testing of the mother is recommended prior to treatment where possible.
- Infants (under 1 year) who have remained in hospital since birth and were born <28/40 gestation OR had a birth weight <1kg and who are non-immune or have a varicella IgG level <150mIU
- Infants with severe congenital or underlying conditions requiring prolonged intensive or special care during the first year of life and who are non-immune or have a varicella IgG level <150mIU/ml. In these cases oral or IV aciclovir (dose as per BNF-c, attenuation of infection/PEP ) can be considered as an alternative to VZIG after exposure.
Where the infant is over 4 weeks old (regardless of gestation at birth), oral aciclovir is the preferred option unless contraindicated. Aciclovir commences on day 7 post exposure (dose as per BNF-c, attenuation of infection/PEP ). If contraindicated, VZIG should be given.
Around 50% of infants given VZIG will still develop chicken pox but the course is likely to be milder. Parents should be asked to bring the infant to hospital if symptoms or signs of chickenpox develop. The incubation period of chickenpox in infants who have received VZIG can be up to 28 days.
All 3 criteria must be met
1. Significant Exposure
This is defined as exposure to someone who has chicken pox or disseminated herpes zoster or exposed herpes zoster lesions or an immunosuppressed person with herpes zoster on any part of the body
and
Is a household contact or the contact involves being in the same room for 15 minutes or more or has had face to face contact e.g. conversation or an immunosuppressed contact on an open ward
2. Timing
Prophylaxis is required if the contact is between:
- 24 hours before the onset of rash until crusting for chickenpox and disseminated herpes zoster
OR - or day of onset of rash until crusting for localised zoster infection.
3. Increased Risk
Increased risk patients include:
- Severe primary immunodeficiency
- Current or within 6 months of treatment for malignancy with chemotherapy or radiotherapy
- All solid organ transplant patients on immunosuppressive therapy
- All bone marrow transplant patients up to 12 months after immunosuppressive therapy – may be longer (discuss with patient’s medical team) if not on immunoglobulin replacement therapy
- All patients receiving systemic high dose steroids or who have received them in the past 3 months (prednisolone ≥2mg/kg/day for at least 1 week or ≥1mg/kg/day for at least 28 days)
- HIV patients (unless stable viral suppression on ART with good CD4 count – discuss with responsible ID consultant)
- Patients on other immunosuppressive drugs for e.g. inflammatory bowel disease, rheumatological conditions or have been in last 6 months
- UKHSA.Guidelines on post exposure prophylaxis for varicella/shingles , April 2022
- BNFc Treatment summary: Herpesvirus infections.
- Public Health England. Chickenpox: public health management and guidance.
- Public Health England. Guidance on the use and ordering of varicella zoster immunoglobulin (VZIG).
- Public Health England. Updated guidelines on post exposure prophylaxis (PEP) for varicella/shingles..
- BMJ Best Practice. Acute varicella zoster
- NICE Clinical Knowledge Summaries. Chickenpox
- YC, Lin TY, Lin YJ, Lien RI, Chou YH. Prophylaxis of intravenous immunoglobulin and acyclovir in perinatal varicella. Eur J Pediatr 2001; 160: 91–4
- Klassen TP et al, 2005. Acyclovir for treating varicella in otherwise healthy children and adolescents. Cochrane Database of Systematic Reviews
- STURGEON, J.P., et al. Going Out on a Limb: Do Not Delay Diagnosis of Necrotizing Fasciitis in Varicella Infection. Pediatric emergency care 2015; 31(7), pp. 503-507.
- KIM, S.R., et al. Varicella zoster: an update on current treatment options and future perspectives. Expert opinion on pharmacotherapy 2014; 15(1), pp. 61-71. 13.
- Asano, Y. et al. Postexposure prophylaxis of varicella in family contact by oral acyclovir. Pediatrics 1993, 92(2 I), pp. 219–222.
Last reviewed: 21 July 2022
Next review: 21 February 2025
Author(s): Kirsten Wallace
Version: 1
Approved By: Antimicrobial Utilisation Committee

